Biomarkers of environmental marine pollution for NACCP process along Calabrian coasts.
Colica Carmela,a* Vecchio Immacolata,b Strongoli Maria Concetta,b Ventrice Domenica,c Stefanizzi Francesca,d Marra Rosario,b Iannone Michelangelo,b Mollace Vincenzoe,f
a Institute of Molecular Bioimaging and Physiology, National Research Council, Organizational Support Unit (IBFM-CNR-UOS) of Germaneto, 88100 Catanzaro, Italy
b Institute of Neurological Sciences, National Research Council, Organizational Support Unit (ISN-CNR-UOS) of Roccelletta di Borgia, 88021 Catanzaro, Italy
c Regional Agency for Environmental Protection of Calabria (A.R.P.A.Cal), 88100 Catanzaro, Italy
d Research Center for Oliviculture and the Olearia Industry, CRA, C / by Li Rocchi, 87036 Rende, Cosenza, Italy
e Department of Health Sciences, University "Magna Græcia" of Catanzaro, 88100 Germaneto, Catanzaro, Italy
f Interregional Research Center for Food Safety & Health (IRC-FSH),University of Catanzaro "Magna Græcia", 88100 Germaneto, Catanzaro, Italy
Introduction
Non-communicable diseases (NCDs) are the leading cause of death worldwide, causing a number of deaths that exceeds the sum of all other diseases and a more significant impact of these forms of disease falls on low-and middle-income populations. NCDs have reached epidemic proportions, but they could easily be avoided and significantly reduced by the implementation of preventive policies to eliminate or reduce risk factors.
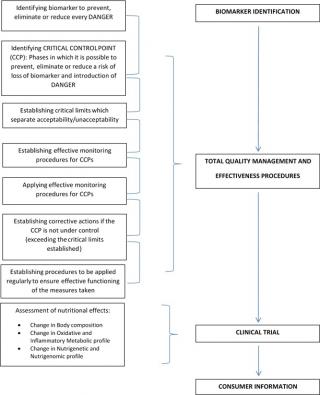
Presently, we do not only need safe food but food that can help the consumer maintain a good state of health and prevent NCDs, the identification of new biomarkers is required to apply the NACCP process, for total quality management (TMQ), and high nutritional levels.1 (Figure 1).
Figure 1. The NACCP process
The present era is called Anthropocene, due to the impact of human activities on the entire terrestrial ecosystem. The territorial, structural and climate changes operated by humans have consequences on all other living organisms.2 When a toxic compound penetrates an ecosystem, it can cause a number of alterations at different levels of structural complexity, ranging from molecular damage to modifications at the level of organisms, populations or communities. Some of these living organisms can provide information about the environment in which they live, therefore, they are referred to as sentinel organisms.3 In these organisms, some parameters are well detectable as biological markers or indicators, providing information on the effects of the contaminants on biological systems.
The biomarkers or stress indexes can be defined as alterations, induced by a contaminant, at the level of molecular or cellular components, of a structure or function, which can be detected and quantified in a sentinel organism. Simultaneously to the negative impact of the pollutant, the organism develops adaptive responses to stress that aim to bring to a state of homeostasis.
The term biomarker has been defined by the National Academy of Sciences in the USA as follows: “A biomarker is a xenobiotically induced variation in cellular or biochemical components or processes, structures, or functions that is measurable in a biological system or sample”.4
Such responses tend to decrease the toxic effect of the pollutant through the involvement of multi-enzymatic systems such as metallothioneins,5 acetylcholinesterase6 and cytochromes.7 These enzymatic systems can detoxify the organism entirely or in part.
Aquatic organisms are subjected to a different kind of stress due to human activities. It’s difficult to evaluate the impact of anthropogenic pollution on the communities belonging to aquatic ecosystem,8, 9 and investigations involving chemical analysis only, are not sufficient to study problems of this magnitude, since pollutants can have deleterious effects on living organisms even when present in low concentrations.10 Considering these limits, recent environmental monitoring programs utilize “biomarkers”11 as a methodological approach, in order to evaluate the organism’s response to environmental stress of chemical or physical nature.12
Because of their filtering habits, bivalve molluscs represent a useful sentinel species for assessing marine contamination.13, 14, 15,16
Mytilus galloprovincialis is a specie widely used as a bio-indicator organism in environmental monitoring, due to the numerous advantages it offers,17 not least its high performance in histological studies and biochemical analysis.18
Digestive gland of this cosmopolitan bivalve species has already been largely used in several previous marine studies. In fact, in the digestive gland the mussels accumulate high levels of heavy metals, because this organ represents their most important detoxifying system.19,20
Metallothioneins are inducible proteins and represent a suitable potential biomarker of metal environmental pollution.21,22 It is widely known that these proteins have biological functions related to homeostatic metals control23 and detoxification of excess metals.24, 25, 26
Molecular biology recent advances allowed a precise characterization of different protein isoforms of metallothioneins27,28,29,30 and clarified their role in homeostatic and detoxification mechanisms31, 32 also identifying the expression of specific genes associated with cellular functions.33, 34, 35
Different gene isoforms in Mytilus galloprovincialis have been identified.36 It has been documented in literature that essential metals exposure, such as zinc (Zn), induces a rapid gene MT10 response,37 while MT20 gene isoform is specifically induced by non-essential metals, such as copper (Cu), and cadmium (Cd).38
AChE enzyme is responsible for hydrolyzing the neurotransmitter acetylcholine into choline and acetic acid.39 AChE is usually located in the membranes (e.g. of erythrocytes) of vertebrates and non-vertebrates; the enzyme controls ionic currents in excitable membranes and plays an essential role in nerve conduction processes at the neuromuscular junction. The inhibition of AChE is linked directly with the mechanism of toxic action of organophosphate and organochlorinated insecticides, by irreversible or reversible binding to the catalytic site of the enzyme and potentiation of cholinergic effects.40 Marine bivalves such as clams, oysters and mussels are widely used as bioindicators of contamination in the monitoring of pollutant effects.41,42,43 However, only a few studies have examined the effects of organophosphate insecticides in aquatic invertebrates44,45,46 and particularly in mussels, which are widely used in pollution monitoring programs.47 Apart from the insecticides, a few other contaminants, including cadmium, mercury, lead and copper were found to show anticholinesterase activity.48
It’s well known that the CYP1A subfamily plays a key role in the biotransformation of contaminants like dioxins, furans, polychlorinated biphenyls and polycyclic aromatic hydrocarbons.49 The induction of CYP1A is triggered via the cytosolic aryl hydrocarbon (Ah) receptor which is activated by exposure of organisms to such pollutants.50 The measurement of the induction of CYP1A in terms of 7-ethoxyresorufin O-deethylase (EROD) activities is utilized as a potential biomarker for marine pollution monitoring.51 Extensive studies were carried out to investigate changes on EROD in the freshwater bivalves, exposed to different pollutants (Arochlor 1260, CB-153 and CB-126, pp¢ DDT, Chlorpyrifos, Carbaryl) at laboratory conditions.52
The aim of this work is to provide further data for the evaluation of the ecotoxicological status of the Calabrian coastal waters using assays of metallothionein gene expressions, acetylcholinesterase and cytochrome P450 enzyme activities, using, as a target organ, the digestive gland of a population of transplanted mussels.
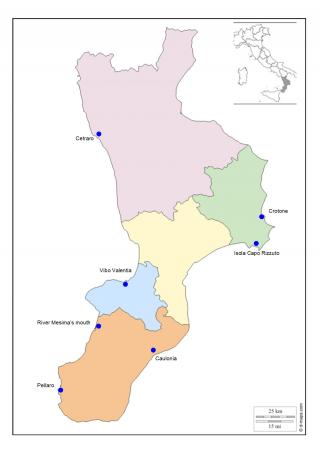
In this work, we describe the results obtained using a range of biomarkers in transplanted mussels in pilot monitoring stations already identified by the National Coastal Waters Monitoring Programme (Law 979/82). For this study, seven Calabrian seaside locations have been identified: four situated on the Ionic coast (Caulonia, Crotone, Isola Capo Rizzuto, and Pellaro) and three on the Tyrrhenian coast (Cetraro, river Mesima’s mouth, and Vibo Marina) (Figure 2, Table 1).
Figure 2. Study areas along the coastline of Calabria, Italy
We have considered critical areas Crotone and river Mesima’s mouth; and white areas Cetraro and Isola Capo Rizzuto.
Crotone was chosen because it was an important industrial site (Pertusola – Montedison), river Mesima’s mouth was chosen because it has been an intensive agricultural area.
Cetraro and Isola Capo Rizzuto were chosen for their statement as protected marine areas.
The selection of the surveyed areas (sampling areas) was carried out on the basis of the knowledge of the different territorial realities and of the results obtained from the previous monitoring programs. The aim is to identify areas subject to specific stresses, critical areas, and areas that are subjected to inferior anthropic impacts, thus assuming the function of control areas or white areas. The latter have been identified mainly within Marine Protected Areas (AMPs).53
Materials and Methods
Study areas and samples
In the summer, mussels belonging to the species Mytilus galloprovincialis were acquired from a mussel farming facility with a long line system, situated in the coastal waters close to Crotone. Seven aliquots (about 10 Kg) were positioned, one at each of the seven study areas [see table 1], with a further aliquot used as a control sample (non-transplanted mussels). All the transplanted mussels were left to stabilize for twelve weeks according to the Mussel Watch protocol;54 the control, instead, were immediately processed.
From a pool of 30 individuals for each monitoring station and control aliquot, the digestive glands were separated from other soft tissues and, for each aliquot, chemical and molecular analyses were carried out. The biomarkers analyzed, assayed according to standardized methods reported in the literature, were respectively: metallothionein (MT10 and MT20) gene expressions,55 and acetylcholinesterase56 and cytochrome P450 activities.57
RNA extraction, cDNA synthesis, quantitative real time PCR analysis of MT genes
Total RNA has been obtained from 30 mg of pooled digestive glands by RNA aqueous-Micro Kit (Ambion); RNA clearness and concentrations have been established by absorbance (A260/A280) measurement. cDNA has been synthesized from 0,5-1 µg of RNA by High Capacity RNA to cDNA Kit (Applied Biosystems), and gene expression of MT10 (GenBank n° AY 566248) and MT20 (GenBank n° AY 566247) mRNA is quantitatively measured by termocycler real-time PCR (Biorad, mod.IQ5), with SYBR® Green PCR Master Mix (Applied Biosystems) and primers: MT10: FW:5’-GGGCGCCGACTGTAAATGTTC-3’; MT10: RW:5’-CACGTTGAAGGYCCTGTACACC-3’; MT20: FW:5’-TGTGAAAGTGGCTGCGGA-3’; MT20: RW:5’-GTACAGCCACATCCACACGC-3’.
Gene targets MT10 and MT20 mRNA expression levels are normalized with a 18S rRNA fragment (GenBank n° L33452) as housekeeping in presence of primers: 18S FW: 5’-TCGATGGTACGTGATATGCC-3’; 18S RW: 5’-CGTTTCTCATGCTCCCTCTC-3’.
Enzymatic activity of acetylcholinesterase
Using the colorimetric method first developed by Ellman et al. (1961),58 the activity of AChE can be measured in digestive glands of mussels. A synthetic substrate for AChE, acetylthiocholine iodide (AtCh), is broken down to thiocholine and acetate. When let thiocholine reacts with dithiobisnitrobenzoate (DTNB), 2-nitrobenzoate-5-mercaptothiocholine and 5-thio-2-nitrobenzoate are produced. The latter has a yellow color that is detectable by a spectrophotometer at 405 nm. The intensity of the yellow color reflects the activity of AChE. The method has been opportunely modified to microplate.59 Prior to AChE biochemical analysis, the tissue was ground in (1:1) Tris-HCL 10 mM pH 7.6, KCL 0.15M, Sucrose 0.5 M, Aprotinin 16.8 mU buffer. Each sample obtained by 1 g of tissue homogenization in 50 mM TRIS pH 7.5, 1 mM EDTA, and 150 mM NaCl buffer, incubated on ice for 20 min, has been centrifuged at 9.000 g for 30 min at 4°C. The supernatant was removed and used to determine AChE activity. Protein content has been spectrophotometrically (562 nm) determined by BCA assay as previously described.60
Briefly: 50 µl of three different concentration of each sample for well reacted with 200 µl of the assay buffer: AtCh 0.5 mM, DTNB 0.3 mM, and phosphate buffered saline (PBS) 0.1 M pH 7.2. Reading assay has been performed for 30 min. The ∆OD/min has been calculated from the linear portion of the curve after the substrate autohydrolysis subtraction. Results are expressed as triplicate mean values +/- mean standard error (S.E.M.) normalized for Torpedo Californica AChE content and expressed as µmoles (micromoles) of substrate produced for min for mg of protein.
Enzymatic activity of cytochrome P450
Enzymatic activity of the CYP450, CYP1A1 isoform has been determined by a fluorimetric Kit (IZKUS Environment). EROD61 activity has been determined on 50 µl of supernatant (obtained by 1 g of tissue homogenization in 50 mM TRIS pH 7.5, 1 mM EDTA, and 150 nM NaCl buffer, and centrifuged at 9000 g for 20 min., 4°C) and 10 μΜ NADPH in 100 mM of KH2PO4, with 7-ethoxyresorufin, to start the reaction. The assay was carried out for 40 minutes by a fluorimeter reader (Jasco, FP-920). The ∆OD/min has been calculated from the linear portion of the curve. EROD activity has been determined in tissue extracts. Results are expressed as triplicate mean values + mean standard error (S.E.M.) and expressed as picomoles (pmoles) of substrate produced for min for mg of protein. This method agrees with ISO standard for PAH and PCBH water pollution determination.
Chemical analysis
Heavy metals analysis was carried out on lyophilised tissue. Samples were digested with HNO3 using a microwave (Ethos Touch Control, Milestone) oven with electronic temperature and pressure controls, in order to avoid loss of the analytes during the heating process. The concentration of individual substance was determined using optical ICP62 (mod. Optima 2100 DV, Perkin Elmer). The compounds of organic nature (carbamates, organic phosphates, halogenated hydrocarbons) were determined using gas chromatography linked to mass spectroscopy.63
Statistical analysis
The statistical analysis of data was done using one-way analysis of variance (ANOVA) followed by Student’s T post-hoc test. Correlation coefficients (R) were determined using regression analysis at a significant level of 95% confidence intervals on mean values. Means were referred to three biological repeats for each set of three independent experiments + mean standard error (S.E.M.). The significance of results was ascertained at P value: p<0.05.
Results
Levels of expression of the MT10 and MT20 genes
Data from gene expression of MT10 and MT20 mRNAs, obtained by experimentation with Q-PCR, was normalized against 18S ribosomal RNA and reported as Normalized Fold Expression, compared to the control, which is reported as a value of 1.
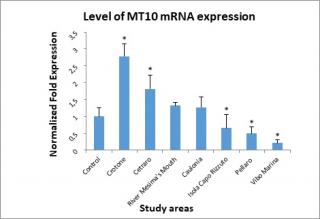
Figure 3 shows the level of MT10 mRNA expression. The highest levels of MT10 mRNA expression were found, in the order, in Crotone and Cetraro monitoring stations. Levels of gene expression slightly above the control were found in the monitoring stations of river Mesima’s mouth and Caulonia. Lower levels than to control were found in Isola Capo Rizzuto, Pellaro, and Vibo Marina areas.
Figure 3. Levels of Metallothionein (MT10) mRNA determined in the digestive gland of mussels.
On the basis of the statistical test used to analyze the experimental data, the value observed in river Mesima’s mouth and Caulonia stations was not considered statistically significant.
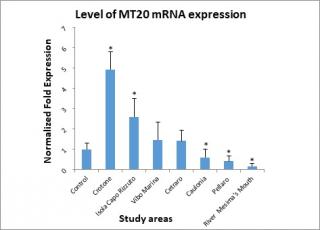
Figure 4 shows the level of MT20 mRNA expression. Mussels positioned at the Crotone monitoring station showed the highest level of expression, followed by Isola Capo Rizzuto. At Vibo Marina and Cetraro were found levels of expression slightly above the control, while in the remaining three study areas: Caulonia, Pellaro, and river Mesima’s mouth were measured the lowest expression’s levels of this metallothionein.
Figure 4. Levels of Metallothionein (MT20) mRNA determined in the digestive gland of mussels.
On the basis of the statistical test used to analyze the experimental data, the value observed in Cetraro and Vibo Marina stations was not considered statistically significant.
2. Acetylcholinesterase enzyme activity
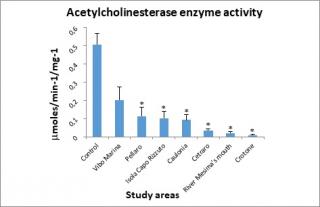
Figure 5 shows the data for acetylcholinesterase enzyme activity. Values obtained are lower than the control sample in all study areas, exceeding the highest threshold (20% inhibition) used by the U.S. EPA (1998)64 for the definition of “biologically significant inhibition” for acetylcholinesterase activity.
Figure 5. Acetylcholinesterase (AChE) enzyme activity determined in the digestive gland of mussels.
At each station, the AChE activity was less than control but, by the statistical test used to analyze the experimental data, the value observed in Vibo Marina station was not considered statistically significant.
3. Cytochrome P450 enzyme activity
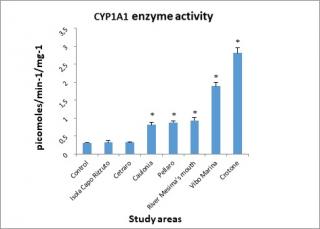
Figure 6 shows the results of the determination of enzyme activity of the cytochrome P450 isoform CYP1A1. Results show a marked increase in CYP1A1 activity in all sites.
Figure 6. Cytochrome P450 (CYP1A1) enzyme activity determined in the digestive gland of mussels.
However, for that concern the values observed in Cetraro and Isola Capo Rizzuto areas, by the statistical test used to analyze the experimental data, these were not considered statistically significant.
Microelements analysis
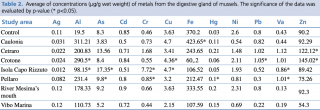
Table 2 shows the concentration (expressed as μg/g wet weight) of microelements, such as heavy metals and metalloids, determined in the digestive gland of Mytilus galloprovincialis in the seven study areas and control sample. In all stations examined, the concentrations of the inorganic chemical elements measured differed more or less markedly from the control sample. Considering the concentration values higher than the control, we note that: Ag concentrations were found to be slightly higher than control (0.11 μg/g w.w) in Vibo Marina and river Mesima’s mouth stations (being 0.12 μg/g w.w in both stations); Al concentrations were found to be considerably higher than control (19.5 μg/g w.w) in all the stations examined (with values ranging from 98.15 μg/g w.w in Isola Capo Rizzuto to 290.5 μg/g w.w in Crotone); As concentrations were found to be slightly higher than control (8.3 μg/g w.w) in Crotone, river Mesima’s mouth, and Pellaro stations (8.64, 9.2, and 9.8 μg/g w.w respectively), and notably higher than control in Cetraro and Isola Capo Rizzuto stations
(13.56 and 17.35 μg/g w.w respectively); Cd concentration was found to be slightly higher than control (0.85 μg/g w.w) only in river Mesima’s mouth station (0.9 μg/g w.w); Cr concentrations were found to be slightly higher than control (0.46 μg/g w.w) in Crotone, river Mesima’s mouth, Caulonia, and Pellaro stations (0.55, 0.66, 0.73, and 0.85 μg/g w.w respectively), notably higher than control in Cetraro station (1.68 μg/g w.w), and considerably higher than control in Isola Capo Rizzuto station (7.72 μg/g w.w); Cu concentrations were found to be slightly higher than control (3.63 μg/g w.w) in Crotone (4.36 μg/g w.w), Caulonia, and Isola Capo Rizzuto stations (being 4.7 μg/g w.w in both stations); Fe concentration was found to be higher than control (370.2 μg/g w.w) only in Caulonia station (423.65 μg/g w.w); Hg concentrations were found higher than control (0.03 μg/g w.w) in all the station examined, precisely: slightly higher in Isola Capo Rizzuto and Crotone stations (0.05 and 0.06 μg/g w.w respectively), notably higher in Pellaro, Caulonia, and Vibo Marina stations (0.1, 0.11, and 0.15 μg/g w.w respectively), considerably higher in river Mesima’s mouth and Cetraro stations (0.2 and 0.21 μg/g w.w respectively); none of the stations tested were found to have Ni concentrations higher than control; Pb concentrations were found to be slightly higher than control (0.8 μg/g w.w) in Caulonia station (0.82 μg/g w.w), and notably higher than control in Cetraro and Crotone (1.02 and 1.05 μg/g w.w respectively); Va concentrations were found to be slightly higher than control (0.43 μg/g w.w) in Caulonia station (0.44 μg/g w.w), notably higher than control in Isola Capo Rizzuto station (0.86 μg/g w.w), and considerably higher than control in Pellaro, Crotone (1.01 μg/g w.w in both stations), and Cetraro (1.12 μg/g w.w); finally, Zn concentrations were found to be slightly higher than control (90.2 μg/g w.w) in Caulonia and river Mesima’s mouth stations (92.29 and 92.3 μg/g w.w respectively), and notably higher than control in Cetraro and Crotone stations (122.12 and 145.02 μg/g w.w respectively).
Organic contaminants
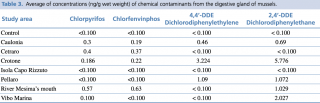
Regarding the analyzed organic compounds, the highest concentration of Chlorpyrifos and Chlorfenvinphos (banned by the European Union since 2003) compounds were detected at the river Mesima’s mouth station. Meanwhile the concentration of 4,4'-Dichlorodiphenylethylene (4,4'DDE) was below the threshold value and the concentration of 2,4'-Dichlorodiphenylethane (2,4'DDE) was significantly above this value. In regards to Pellaro, while the concentrations of Chlorpyrifos and Chlorfenvinphos was below the threshold value, the concentrations of 4,4’-DDE and 2,4’-DDE was significantly above this value. Crotone showed the concentrations of Chlorpyrifos and Chlorfenvinphos significantly above the threshold value, and the highest concentration of 4,4’-DDE and 2,4’-DDE. In Caulonia the concentrations of both organophosphate and organochlorinated compounds were significantly higher than control. Instead, in Isola Capo Rizzuto the levels of all four compounds were below the threshold value. Lastly, Cetraro showed the concentrations of organophosphate compounds significantly higher than control, while the concentration of organochlorinated compounds were below the threshold value. (Table 3).
Discussion
The results obtained from the assays carried out in the present study confirmed the validity of proteins (MT10 and MT20), and enzymes (AChE and CYP1A1) taken into account as pollutant biomarkers.
As described in the literature, the MT10 gene expression is mainly influenced by zinc.65 Elevated expression values of this biomarker were detected in mussels transplanted in the Cetraro (1.82 Normalized Fold Expression) and Crotone (2.77 Normalized Fold Expression) study areas.
The highest level of MT10 gene expression, as shown in the Crotone area, agrees with the highest concentration of zinc found by chemical analysis (145.02 μg/g) and could be ascribed to chemical contamination due to industrial activity which operated for over 50 years in the Crotone area.
The most important industrial factories were: the Pertusola South and the Montedison. The Pertusola, shut down in 1999, operated zinc sulphide producing as discarge zinc ferrites, lead, copper and cadmium. In the beginning, the Montedison produced nitrogen and phosphorus compounds to phosphoric acid generation and then, in 1993, zeolites to cleanser trade.
Cetraro study area also showed high values of MT10 mRNA expression correlated to the presence of elevated zinc value (122.12 μg/g), probably due to the mineral site composition.
Regarding the gene isoform MT20, the highest levels of expression were found in the study areas: Crotone (4.92 Normalized Fold Expression) and Isola Capo Rizzuto (2.59 Normalized Fold Expression).
While, with regard to Crotone, motivation is always to be attributed to the presence of Pertusola and Montedison (since, after the disposal, the area that housed them was never reclaimed), with regard to Isola Capo Rizzuto the motivation could be, as for Cetraro, the composition of minerals present in that area.
Furthermore, some authors, such as Lavradas et al. (2016), have found significant correlations between the contemporary presence of metals and metalloids in the aquatic environment and increased expression of metallothioneins in the digestive gland of mussels,66 this could explain the high expression of MT10 at Cetraro and MT20 at Isola Capo Rizzuto. In fact, in both of these sites, the highest levels of As in the digestive glands of the studied mussels were found, this agrees with the geomorphological nature of these two places, where the rocks exhibit a high percentage of this metalloid in their composition.67
Many authors note that this isoform is Cd specific,68 but in this study the concentrations of Cd found were not thought to be high enough to induce a significant response in the MT20 gene. Instead, it’s plausible that the higher levels of expression found in the Crotone and Isola Capo Rizzuto areas could have been induced by a mixture of different heavy metals, which could have a synergistic effect.69 Concerning Crotone, the increased gene expression of MT20 may depend on the increased concentration of Pb.70 The MT20 gene response, seen in the mussels transplanted to the Isola Capo Rizzuto area, could be attributable to the elevated concentrations of Cr found in the digestive glands (7.72 μg/g).71
In the literature there are many evidences of increased gene expression of some metallothionein stimulated by Al, especially in the liver of mammals and in insects,72,73 but we didn’t find any studies on the correlation between Al and MT10 or MT20 in molluscs. Therefore, despite the Al levels in all stations being markedly higher than control, we must assume that the metallothioneins weighed in our study are not sensitive to its action, so they can’t be considered pollutant biomarkers for this metal.
Regarding acetylcholinesterase,74 it is amply reported in the literature that enzyme activity tends to decrease when the organism is exposed to organophosphate and carbamate compounds, used as pesticides,75 lubricants, fuel additives.76 Other classes of environmental contaminants, including heavy metals,77 also determine a reduction in enzyme activity.
From the chemical data in our possession, a correlation was observed between the concentrations of organophosphate compounds considered in our study and AChE activity. In fact, at all stations the AChE activity was considerably lower than control, especially in those where the highest concentrations of organophosphate compounds were detected, specifically in Crotone, river Mesima’s mouth, and Cetraro stations, where the lower levels of AChE activity were founded. Some authors reported that AChE activity can be modulated by trace metals (Cd, Cu, Hg, Zn) or natural factors (seawater temperature, biotoxins or cyanobacteria in mussel tissues).78-81 The moderate inhibition values of AChE activity observed in Caulonia (0.0932μmoles/min-1/mg-1), Isola Capo Rizzuto (0.102μmoles/min-1/mg-1), and Pellaro (0.114 μmoles/min-1/mg-1) could be explained by concomitant factors of different origins, such as intensive agricultural practices in Caulonia, natural composition of the rocks in Isola Capo Rizzuto, and urban pollution (as a consequence of geographic proximity to Reggio Calabria, where is located an important port to and from Sicily) in Pellaro.
The reduction in AChE activity, in the Crotone (0.01 μmoles/min-1/mg-1), river Mesima’s mouth (0.0199 μmoles/min-1/mg-1), and Cetraro (0.0349 μmoles/min-1/mg-1) sites could be hypothesised as the result of a synergistic effect between organophosphate compounds (Chlorpyrifos: 0.186 ng/g, 0.57 ng/g and 0.4 ng/g; Chlorfenvinfos: 0.22 ng/g, 0.63 ng/g and 0.37 ng/g respectively) and zinc levels (145.02 μg/g, 92.3 μg/g and 122,12 μg/g respectively) measured.
Enzyme activity of the cytochrome P450 isoform CYP1A1, as noted in the literature,82 is induced by the classes of the most widely distributed contaminants, the polycyclic aromatic hydrocarbons (PAH),83 the nitro-polycyclic aromatic hydrocarbons (NPAH),84 the polychlorobiphenyls (PCB),85 dioxins (TCDD)86 and some pesticides87 and altered by heavy metals such as Cu, Cd and Hg.88
The enzymatic response seen in the Crotone (2.81 pmoles/min-1/mg-1) and Vibo Marina (1.89 pmoles/min-1/mg-1) areas could be correlated with the concentration of 2,4 DDE (5.776 ng/g and 2.027 ng/g respectively), belonging to the halogenated hydrocarbons, of which the inductive effect on CYP1A1 activity is well known.89
The homogeneity of values measured in river Mesima’s mouth (0.938 pmoles/min-1/mg-1), Pellaro (0.878 pmoles/min-1/mg-1), and Caulonia (0.812 pmoles/min-1/mg-1) reflected the levels of the considered contaminants (see tables 2 and 3).
Conclusions
This paper presents an overview of the significance of the use of molecular biomarkers as diagnostic and prognostic tools for marine pollution monitoring, useful for NACCP process.
Furthermore, biomarkers, inside the most recent laws in this sector (Directive 2000/60/EC, Lgs. D. 152/06 and i.s.m.), can be widely used in an integrated evaluation system of the marine environment quality. Among the various types of biomarkers, the following have received particular attention, because of their peculiar responsiveness, as extensively reported in literature: metallothioneins (MT10, MT20) expression, acetylcholinesterase activity and cytochrome CYP1A1/P450 induction. These biomarkers are being used to evaluate exposure of mussels, a sentinel marine organism, to the effect of various contaminants (organic xenobiotics and metals) using different molecular approaches (biochemical assays, fluorimetric measurement, polymerase chain reaction). The selected biomarkers indicate that the organism has been exposed to pollutants (exposure biomarkers) and that the magnitude of the organism’s response is proportional to the pollutant’s levels (effect biomarkers or biomarkers of stress). Results from this work give us more elements to evaluate the study areas under the bioecological impact, as prescribed by the new methodological approaches for the study of coastal marine environments. The use of biomarkers in an integrated analysis of the quality of the marine environment is by now fully accepted. This consideration, strongly recommended by the Scientific Community, should support the definitive introduction of biomarkers as routine monitoring parameters in programmes for the monitoring and control of coastal marine environments.
The identification of these biomarkers for the NACCP process,90 applied to the marine sector, is a change of perspective in the prevention of NCDs, through a comprehensive and integrated system of information, as indicated by the vision of predictive medicine, preventive, personalized and participatory.
Acknowledgements
Special thanks to Antonio Macrì, and Domenico Saturnino (ISN/CNR U.O.S. Roccelletta di Borgia), Emanuele Vizza (Regional Agency for Environmental Protection of Calabria A.R.P.A.Cal) for their technical support, Emilio Cellini (A.R.P.A.Cal) for his coordinating role, and Ruby Emma Baricchi for having revisited the language of manuscript.
Authors’ Contributions
Carmela Colica, Immacolata Vecchio, and Maria Concetta Strongoli contributed equally to this project. Carmela Colica conceived and designed the experiments. Immacolata Vecchio took care of statistical data processing and the drawing of the graphs. Carmela Colica, Immacolata Vecchio, and Maria Concetta Strongoli contributed to analyzation and interpretation of the data, and to the writing and revision of the manuscript. Domenica Ventrice performed the chemical assays. Francesca Stefanizzi performed the PCR experiments. Rosario Marra, and Michelangelo Iannone provided technical support. Carmela Colica had primary responsibility for the final content. Vincenzo Mollace funded the study. All the authors read and approved the final manuscript.
References
- Food Safety and Nutritional Quality for the Prevention of Non Communicable Diseases: The Nutrient, Hazard Analysis and Critical Control Point Process (NACCP). Di Renzo L, Colica C, Carraro A, Cenci Goga B, Marsella LT, Botta R, Colombo ML, Gratteri S, Chang TF, Droli M, Sarlo F, De Lorenzo A. J Transl Med. 2015 Apr 23; 13:128. doi: 10.1186/s12967-015-0484-2.
- Stebbing N. Therapeutic Considerations for Use of Immunomodulators in the Treatment of Ataxia-Telangiectasia. Kroc Found Ser. 1985; 19:339-52.
- O'Brien DJ, Kaneene JB, Poppenga RH. The Use of Mammals as Sentinels for Human Exposure to Toxic Contaminants in the Environment. Environ Health Perspect. 1993 Mar; 99:351-68.
- National Research Council Committee on Biological Markers. Biological Markers in Environmental Health Research. Environ Health Perspect. 1987 Oct; 74:3-9.
- Davis SR, Cousins RJ. Metallothionein Expression in Animals: A Physiological Perspective on Function. J Nutr. 2000 May; 130(5):1085-8.
- Yadav A, Gopesh A, Pandey RS, Rai DK, Sharma B. Acetylcholinesterase: A Potential Biochemical Indicator for Biomonitoring of Fertilizer Industry Effluent Toxicity in Freshwater Teleost, Channa Striatus. Ecotoxicology. 2009 Apr; 18(3):325-33. doi: 10.1007/s10646-008-0286-x.
- Whitlock JP Jr. Induction of Cytochrome P4501A1. Annu Rev Pharmacol Toxicol. 1999; 39:103-25.
- Galloway TS. Biomarkers in Environmental and Human Health Risk Assessment. Mar Pollut Bull. 2006; 53(10-12):606-13.
- Sarkar A, Ray D, Shrivastava AN, Sarker S. Molecular Biomarkers: Their Significance and Application in Marine Pollution Monitoring. Ecotoxicology. 2006 May; 15(4):333-40.
- Zaharia C. Evaluation of Environmental Impact Produced by Different Economic Activities with the Global Pollution Index. Environ Sci Pollut Res Int. 2012 Jul; 19(6):2448-55. doi: 10.1007/s11356-012-0883-3.
- Allen JI, Moore MN. Environmental Prognostics: Is the Current Use of Biomarkers Appropriate for Environmental Risk Evaluation? Mar Environ Res. 2004 Aug-Dec; 58(2-5):227-32.
- Hook SE, Gallagher EP, Batley GE. The Role of Biomarkers in the Assessment of Aquatic Ecosystem Health. Integr Environ Assess Manag. 2014 Jul; 10(3):327-41. doi: 10.1002/ieam.1530.
- Dixon DR, Pruski AM, Dixon LR, Jha AN. Marine Invertebrate Eco-Genotoxicology: A Methodological Overview. Mutagenesis. 2002 Nov;17(6):495-507.
- Galletly BC, Blows MW, Marshall DJ. Genetic Mechanisms of Pollution Resistance in a Marine Invertebrate. Ecol Appl. 2007 Dec; 17(8):2290-7.
- Guerlet E, Vasseur P, Giambérini L. Spatial and Temporal Variations of Biological Responses to Environmental Pollution in the Freshwater Zebra Mussel. Ecotoxicol Environ Saf. 2010 Sep; 73(6):1170-81. doi: 10.1016/j.ecoenv.2010.05.009.
- Moschino V, Delaney E, Meneghetti F, Ros LD. Biomonitoring Approach with Mussel Mytilus Galloprovincialis (Lmk) and Clam Ruditapes Philippinarum (Adams and Reeve, 1850) in the Lagoon of Venice. Environ Monit Assess. 2011 Jun; 177(1-4):649-63. doi: 10.1007/s10661-010-1663-5.
- Regoli F, Frenzilli G, Bocchetti R, Annarumma F, Scarcelli V, Fattorini D, Nigro M. Time-Course Variation of Oxyradical Metabolism, DNA Integrity and Lysosomal Stability in Mussels, Mytilus Galloprovincialis, During a Field Translocation Experiment. Aquat Toxicol. 2004 Jun 10; 68(2):167-78.
- Sureda A, Box A, Tejada S, Blanco A, Caixach J, Deudero S. Biochemical Responses of Mytilus Galloprovincialis as Biomarkers of Acute Environmental Pollution Caused by the Don Pedro Oil Spill (Eivissa Island, Spain). Aquat Toxicol. 2011 Feb; 101(3-4):540-9. doi: 10.1016/j.aquatox.2010.12.011.
- Marigómez I, Soto M, Cajaraville MP, Angulo E, Giamberini L. Cellular and Subcellular Distribution of Metals in Molluscs. Microsc Res Tech. 2002 Mar 1; 56(5):358-92.
- Ng TY, Wang WX. Dynamics of Metal Subcellular Distribution and Its Relationship with Metal Uptake in Marine Mussels. Environ Toxicol Chem. 2005 Sep; 24(9):2365-72.
- Isani G, Andreani G, Kindt M, Carpenè E. Metallothioneins (MTs) in Marine Molluscs. Cell Mol Biol (Noisy-le-grand). 2000 Mar; 46(2):311-30.
- Cosson RP. Bivalve Metallothionein as a Biomarker of Aquatic Ecosystem Pollution by Trace Metals: Limits and Perspectives. Cell Mol Biol (Noisy-le-grand). 2000 Mar; 46(2):295-309.
- Mao H, Wang DH, Yang WX. The Involvement of Metallothionein in the Development of Aquatic Invertebrate. Aquat Toxicol. 2012 Apr; 110-111:208-13. doi: 10.1016/j.aquatox.2012.01.018.
- Bremner I. Interactions Between Metallothionein and Trace Elements. Prog Food Nutr Sci. 1987;11(1):1-37.
- Verlecar XN, Jena KB, Chainy GB. Modulation of Antioxidant Defences in Digestive Gland of Perna Viridis (L.), on Mercury Exposures. Chemosphere. 2008 May; 71(10):1977-85. doi: 10.1016/j.chemosphere.2007.12.014
- Velez C, Galvão P, Longo R, Malm O, Soares AM, Figueira E, Freitas R. Ruditapes Philippinarum and Ruditapes Decussatus under Hg Environmental Contamination. Environ Sci Pollut Res Int. 2015 Aug; 22(15):11890-904. doi: 10.1007/s11356-015-4397-7.
- Lemoine S, Bigot Y, Sellos D, Cosson RP, Laulier M. Metallothionein Isoforms in Mytilus edulis (Mollusca, Bivalvia): Complementary DNA Characterization and Quantification of Expression in Different Organs after Exposure to Cadmium, Zinc, and Copper. Mar Biotechnol (NY). 2000 Mar; 2(2):195-203.
- Soazig L, Marc L. Potential Use of the Levels of the Mrna of a Specific Metallothionein Isoform (MT-20) in Mussel (Mytilus edulis) as a Biomarker of Cadmium Contamination. Mar Pollut Bull. 2003 Nov; 46(11):1450-5.
- Vergani L, Grattarola M, Grasselli E, Dondero F, Viarengo A. Molecular Characterization and Function Analysis of MT-10 and MT-20 Metallothionein Isoforms from Mytilus galloprovincialis. Arch Biochem Biophys. 2007 Sep 1; 465(1):247-53.
- Buico A, Cassino C, Dondero F, Vergani L, Osella D. Radical Scavenging Abilities of Fish MT-A and Mussel MT-10 Metallothionein Isoforms: An ESR Study. J Inorg Biochem. 2008 Apr; 102(4):921-7. doi: 10.1016/j.jinorgbio.2007.12.012.
- Roesijadi G. Metal Transfer as a Mechanism for Metallothionein-Mediated Metal Detoxification. Cell Mol Biol (Noisy-le-grand). 2000 Mar; 46(2):393-405.
- Santovito G, Piccinni E, Boldrin F, Irato P. Comparative Study on Metal Homeostasis and Detoxification in Two Antarctic Teleosts. Comp Biochem Physiol C Toxicol Pharmacol. 2012 May; 155(4):580-6. doi: 10.1016/j.cbpc.2012.01.008.
- Tanguy A1, Mura C, Moraga D. Cloning of a Metallothionein Gene and Characterization of Two Other Cdna Sequences in the Pacific Oyster Crassostrea Gigas (CgMT1). Aquat Toxicol. 2001 Nov 1; 55(1-2):35-47.
- Tanguy A, Moraga D. Cloning and Characterization of a Gene Coding for a Novel Metallothionein in the Pacific Oyster Crassostrea gigas (CgMT2): A Case of Adaptive Response to Metal-Induced Stress? Gene. 2001 Jul 25; 273(1):123-30.
- Takahashi S. Positive and Negative Regulators of the Metallothionein Gene (Review). Mol Med Rep. 2015 Jul; 12(1):795-9. doi: 10.3892/mmr.2015.3459.
- Ceratto N, Dondero F, van de Loo JW, Burlando B, Viarengo A. Cloning and Sequencing of a Novel Metallothionein Gene in Mytilus Galloprovincialis Lam. Comp Biochem Physiol C Toxicol Pharmacol. 2002 Mar; 131(3):217-22.
- Pellerin J, Amiard JC. Comparison of Bioaccumulation of Metals and Induction of Metallothioneins in Two Marine Bivalves (Mytilus edulis and Mya arenaria). Comp Biochem Physiol C Toxicol Pharmacol. 2009 Aug; 150(2):186-95. doi: 10.1016/j.cbpc.2009.04.008.
- Syring RA, Hoexum Brouwer T, Brouwer M. Cloning and Sequencing of Cdnas Encoding for a Novel Copper-Specific Metallothionein and Two Cadmium-Inducible Metallothioneins from the Blue Crab Callinectes sapidus. Comp Biochem Physiol C Toxicol Pharmacol. 2000 Mar; 125(3):325-32.
- Nachmansohn D, Wilson IB. The Enzymic Hydrolysis and Synthesis of Acetylcholine. Adv Enzymol Relat Subj Biochem. 1951; 12:259-339.
- Pope C, Karanth S, Liu J. Pharmacology and Toxicology of Cholinesterase Inhibitors: Uses and Misuses of a Common Mechanism of Action. Environ Toxicol Pharmacol. 2005 May; 19(3):433-46. doi: 10.1016/j.etap.2004.12.048.
- Bebianno MJ, Géret F, Hoarau P, Serafim MA, Coelho MR, Gnassia-Barelli M, Roméo M. Biomarkers in Ruditapes decussatus: A Potential Bioindicator Species. Biomarkers. 2004 Jul-Oct; 9(4-5):305-30.
- Hédouin L, Pringault O, Bustamante P, Fichez R, Warnau M. Validation of Two Tropical Marine Bivalves as Bioindicators of Mining Contamination in the New Caledonia Lagoon: Field Transplantation Experiments. Water Res. 2011 Jan; 45(2):483-96. doi: 10.1016/j.watres.2010.09.002.
- Kristan U, Kanduč T, Osterc A, Šlejkovec Z, Ramšak A, Stibilj V. Assessment of Pollution Level Using Mytilus Galloprovincialis as a Bioindicator Species: The Case of the Gulf of Trieste. Mar Pollut Bull. 2014 Dec 15; 89(1-2):455-63. doi: 10.1016/j.marpolbul.2014.09.046.
- Rubach MN, Baird DJ, Boerwinkel MC, Maund SJ, Roessink I, Van den Brink PJ. Species Traits as Predictors for Intrinsic Sensitivity of Aquatic Invertebrates to the Insecticide Chlorpyrifos. Ecotoxicology. 2012 Oct; 21(7):2088-101. doi: 10.1007/s10646-012-0962-8.
- Pérez E, Blasco J, Solé M. Biomarker Responses to Pollution in Two Invertebrate Species: Scrobicularia plana and Nereis diversicolor from the Cádiz Bay (SW Spain). Mar Environ Res. 2004 Aug-Dec; 58(2-5):275-9.
- Lau PS, Wong HL. Effect of Size, Tissue Parts and Location on Six Biochemical Markers in the Green-Lipped Mussel, Perna viridis. Mar Pollut Bull. 2003 Dec;46(12):1563-72.
- Cajaraville MP, Bebianno MJ, Blasco J, Porte C, Sarasquete C, Viarengo A. The Use of Biomarkers to Assess the Impact of Pollution in Coastal Environments of the Iberian Peninsula: A Practical Approach. Sci Total Environ. 2000 Mar 20; 247(2-3):295-311.
- Lionetto MG, Caricato R, Giordano ME, Pascariello MF, Marinosci L, Schettino T. Integrated Use of Biomarkers (Acetylcholinesterase and Antioxidant Enzymes Activities) in Mytilus galloprovincialis and Mullus barbatus in an Italian Coastal Marine Area. Mar Pollut Bull. 2003 Mar; 46(3):324-30.
- Hawkins SA, Billiard SM, Tabash SP, Brown RS, Hodson PV. Altering Cytochrome P4501A Activity Affects Polycyclic Aromatic Hydrocarbon Metabolism and Toxicity in Rainbow Trout (Oncorhynchus mykiss). Environ Toxicol Chem. 2002 Sep; 21(9):1845-53.
- Mu JL, Wang XH, Jin F, Wang JY, Hong HS. The Role of Cytochrome P4501A Activity Inhibition in Three- to Five-Ringed Polycyclic Aromatic Hydrocarbons Embryotoxicity of Marine Medaka (Oryzias melastigma). Mar Pollut Bull. 2012 Jul; 64(7):1445-51. doi: 10.1016/j.marpolbul.2012.04.007.
- Fatima RA, Ahmad M. Allium Cepa Derived EROD as a Potential Biomarker for the Presence of Certain Pesticides in Water. Chemosphere. 2006 Jan; 62(4):527-37.
- Binelli A, Ricciardi F, Riva C, Provini A. New Evidences for Old Biomarkers: Effects of Several Xenobiotics on EROD and Ache Activities in Zebra Mussel (Dreissena polymorpha). Chemosphere. 2006 Jan; 62(4):510-9.
- Manuale ICRAM – Programma di monitoraggio per il controllo dell’ambiente marino costiero (triennio 2001 – 2003).
- Quaderno ICRAM 2006 – Bioaccumulo in bivalvi - Scheda 1 – Utilizzo dei molluschi bivalvi nel programma di monitoraggio dell’ambiente costiero (Protocollo Mussel Watch).
- Dondero F, Piacentini L, Banni M, Rebelo M, Burlando B, Viarengo A. Quantitative PCR Analysis of Two Molluscan Metallothionein Genes Unveils Differential Expression and Regulation. Gene. 2005 Jan 31; 345(2):259-70.
- Magni P, De Falco G, Falugi C, Franzoni M, Monteverde M, Perrone E, Sgro M, Bolognesi C. Genotoxicity Biomarkers and Acetylcholinesterase Activity in Natural Populations of Mytilus Galloprovincialis Along a Pollution Gradient in the Gulf of Oristano (Sardinia, western Mediterranean). Environ Pollut. 2006 Jul; 142(1):65-72.
- Jonsson H, Sandnes KV, Schiedek D, Schneider R, Grøsvik BE, Goksøyr A. Development of Two Novel CYP-Antibodies and Their Use in a PCB Exposure Experiment with Mytilus Edulis. Mar Environ Res. 2004 Aug-Dec; 58(2-5):655-8.
- Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem Pharmacol. 1961 Jul; 7:88-95.
- Guilhermino L, Celeste Lopes M, Carvalho AP, Soares AM. Inhibition of Acetylcholinesterase Activity as Effect Criterion in Acute Tests with Juvenile Daphnia magna. Chemosphere. 1996 Feb; 32(4):727-38.
- Nielsen M, Braestrup C. The Apparent Target Size of Rat Brain Benzodiazepine Receptor, Acetylcholinesterase, and Pyruvate Kinase is Highly Influenced by Experimental Conditions. J Biol Chem. 1988 Aug 25; 263(24):11900-6.
- van der Oost R, Beyer J, Vermeulen NP. Fish Bioaccumulation and Biomarkers in Environmental Risk Assessment: A Review. Environ Toxicol Pharmacol. 2003 Feb; 13(2):57-149.
- Seco-Gesto EM, Moreda-Piñeiro A, Bermejo-Barrera A, Bermejo-Barrera P. Multi-Element Determination in Raft Mussels by Fast Microwave-Assisted Acid Leaching and Inductively Coupled Plasma-Optical Emission Spectrometry. Talanta. 2007 May 15; 72(3):1178-85. doi: 10.1016/j.talanta.2007.01.009.
- Vidal JL, Vega AB, Arrebola FJ, González-Rodríguez MJ, Sánchez MC, Frenich AG. Trace Determination of Organotin Compounds in Water, Sediment and Mussel Samples by Low-Pressure Gas Chromatography Coupled to Tandem Mass Spectrometry. Rapid Commun Mass Spectrom. 2003; 17(18):2099-106.
- US EPA (1998). US Environmental Protection Agency. Policy Issues Related to the Food Quality Protection Act. Office of Pesticide Program’s Science Policy on the Use of Cholinesterase Inhibition for Risk Assessment of Organophosphate and Carbamate Pesticides. OPP Docket #00560, Fed. Register 63: 214.
- Lemoine S., Bigot Y., Sellos D., Cosson R.P., Laulier M., Metallothionein Isoforms in Mytilus edulis (Mollusca, Bivalvia): Complementary DNA Characterization and Quantification of Expression in Different Organs after Exposure to Cadmium, Zinc, and Copper. Mar Biotechnol (NY). 2000 Mar; 2(2):195-203.
- Lavradas RT, Rocha RC, Bordon IC, Saint'Pierre TD, Godoy JM, Hauser-Davis RA. Differential Metallothionein, Reduced Glutathione and Metal Levels in Perna Perna Mussels in Two Environmentally Impacted Tropical Bays in Southeastern Brazil. Ecotoxicol Environ Saf. 2016 Jul; 129:75-84. doi: 10.1016/j.ecoenv.2016.03.011.
- De Vivo B, Costabile S, and Lima A. Cartografia Geochimica della Calabria. Mem. Descr. Carta Geol d’It. LV (1988), pp. 17-29.
- Barsyte D, White KN, Lovejoy DA. Cloning and Characterization of Metallothionein Cdnas in the Mussel Mytilus edulis L. Digestive Gland. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999 Feb; 122(2):287-96.
- de Los Ríos A, Pérez L, Echavarri-Erasun B, Serrano T, Barbero MC, Ortiz-Zarragoitia M, Orbea A, Juanes JA, Cajaraville MP. Measuring Biological Responses at Different Levels of Organization to Assess the Effects of Diffuse Contamination Derived from Harbour and Industrial Activities in Estuarine Areas. Mar Pollut Bull. 2016 Feb 15; 103(1-2):301-312. doi: 10.1016/j.marpolbul.2015.11.056.
- Poynton HC, Robinson WE, Blalock BJ, Hannigan RE. Correlation of Transcriptomic Responses and Metal Bioaccumulation in Mytilus edulis L. Reveals Early Indicators of Stress. Aquat Toxicol. 2014 Oct; 155:129-41. doi: 10.1016/j.aquatox.2014.06.015.
- Franzellitti S, Viarengo A, Dinelli E, Fabbri E. Molecular and Cellular Effects Induced by Hexavalent Chromium in Mediterranean Mussels. Aquat Toxicol. 2012 Nov 15; 124-125:125-32. doi: 10.1016/j.aquatox.2012.07.011.
- Ghorbel I, Chaabane M, Elwej A, Boudawara O, Abdelhedi S, Jamoussi K, Boudawara T, Zeghal N. Expression of Metallothioneins I and II Related to Oxidative Stress in the Liver of Aluminium-Treated Rats. Arch Physiol Biochem. 2016 Oct; 122(4):214-222.
- Gauthier M, Aras P, Jumarie C, Boily M. Low Dietary Levels of Al, Pb and Cd May Affect the Non-Enzymatic Antioxidant Capacity in Caged Honey Bees (Apis mellifera). Chemosphere. 2016 Feb; 144:848-54. doi: 10.1016/j.chemosphere.2015.09.057.
- Nunes B. The Use of Cholinesterases in Ecotoxicology. Rev Environ Contam Toxicol. 2011; 212:29-59. doi: 10.1007/978-1-4419-8453-1_2.
- Anguiano GA, Amador A, Moreno-Legorreta M, Arcos-Ortega F, Vazquez-Boucard C. Effects of Exposure to Oxamyl, Carbofuran, Dichlorvos, and Lindane on Acetylcholinesterase Activity in the Gills of the Pacific Oyster Crassostrea gigas. Environ Toxicol. 2010 Aug; 25(4):327-32. doi: 10.1002/tox.20491.
- Dyke PH, Sutton M, Wood D, Marshall J. Investigations on the Effect of Chlorine in Lubricating Oil and the Presence of a Diesel Oxidation Catalyst on PCDD/F Releases from an Internal Combustion Engine. Chemosphere. 2007 Apr; 67(7):1275-86.
- Lionetto MG, Caricato R, Giordano ME, Pascariello MF, Marinosci L, Schettino T. Integrated Use of Biomarkers (Acetylcholinesterase and Antioxidant Enzymes Activities) in Mytilus galloprovincialis and Mullus barbatus in an Italian Coastal Marine Area. Mar Pollut Bull. 2003 Mar; 46(3):324-30.
- Najimi, S., Bouhaimi, S., Daubeze, M., Zekhini, A., Pellerin, J., Narbonne, J.F., Moukrim, A., Use of Acetylcholinesterase in Perna Perna and Mytilus galloprovincialis as a Biomarker of Pollution of Agadir Marine Bay (South of Morocco). Bulletin of Environmental Contamination and Toxicology 1997 58, 901e912.
- Dellali, M., Gnassia-Barelli, M., Romeo, M., Aissa, P., The Use of Acetylcholinesterase. Activity in Ruditapes decussatus and Mytilus galloprovincialis in the Biomonitoring of Bizerta Lagoon. Comparative Biochemistry and Physiology 2001 130, 227e235.
- Dailianis, S., Domouhtsidou, G.P., Raftopoulou, E., Kaloyianni, M., Dimitriadis, V.K., Evaluation of Neutral Red Retention Assay, Micronucleus Test, Acetylcholinesterase Activity and a Signal Transduction Molecule (Camp) in Tissues of Mytilus galloprovincialis (L.), in Pollution Monitoring. Marine Environmental Research 2003 56, 443e470.
- Gorbi, S., Lamberti, C.V., Notti, A., Benedetti, M., Fattorini, D., Moltedo, G., Regoli, F., An Ecotoxicological Protocol with Caged Mussels, Mytilus galloprovincialis, for Monitoring the Impact of an Offshore Platform in the Adriatic Sea. Marine Environmental Research 2008 65, 34e49
- Tabrez S, Ahmad M. Cytochrome P450 System as Potential Biomarkers of Certain Toxicants: Comparison between Plant and Animal Models. Environ Monit Assess. 2013 Apr; 185(4):2977-87. doi: 10.1007/s10661-012-2765-z.
- Incardona JP, Day HL, Collier TK, Scholz NL. Developmental Toxicity of 4-Ring Polycyclic Aromatic Hydrocarbons in Zebrafish is Differentially Dependent on AH Receptor Isoforms and Hepatic Cytochrome P4501A Metabolism. Toxicol Appl Pharmacol. 2006 Dec 15; 217(3):308-21.
- Burkina V, Zlabek V, Zamaratskaia G. Clotrimazole, but Not Dexamethasone, Is a Potent In Vitro Inhibitor of Cytochrome P450 Isoforms CYP1A and CYP3A in Rainbow Trout. Chemosphere. 2013 Aug; 92(9):1099-104. doi: 10.1016/j.chemosphere.2013.01.050.
- Koenig S, Fernández P, Solé M. Differences in Cytochrome P450 Enzyme Activities between Fish and Crustacea: Relationship with the Bioaccumulation Patterns of Polychlorobiphenyls (PCBs). Aquat Toxicol. 2012 Feb; 108:11-7 doi: 10.1016/j.aquatox.2011.10.016.
- Marit JS, Weber LP. Persistent Effects on Adult Swim Performance and Energetics in Zebrafish Developmentally Exposed to 2,3,7,8-Tetrachlorodibenzo-P-Dioxin. Aquat Toxicol. 2012 Jan 15; 106-107:131-9. doi: 10.1016/j.aquatox.2011.11.001.
- Katagi T. Bioconcentration, Bioaccumulation, and Metabolism of Pesticides in Aquatic Organisms. Rev Environ Contam Toxicol. 2010; 204:1-132. doi: 10.1007/978-1-4419-1440-8_1.
- Anwar-Mohamed A, Elbekai RH, El-Kadi AO. Regulation of CYP1A1 by Heavy Metals and Consequences for Drug Metabolism. Expert Opin Drug Metab Toxicol. 2009 May; 5(5):501-21. doi: 10.1517/17425250902918302.
- Bebianno MJ, Lopes B, Guerra L, Hoarau P, Ferreira AM. Glutathione S-Tranferases and Cytochrome P450 Activities in Mytilus galloprovincialis from the South Coast of Portugal: Effect of Abiotic Factors. Environ Int. 2007 May; 33(4):550-8.
- Nutrient Analysis Critical Control Point (NACCP): Hazelnut as a Prototype of Nutrigenomic Study. Laura Di Renzo, Alberto Carraro, Daniela Minella, Roberto Botta, Cecilia Contessa, Chiara Sartor, Anna Maria Iacopino, Antonino De Lorenzo. Food and Nutrition Sciences, 2014, 5, 79-88. Published Online January 2014 (http://www.scirp.org/journal/fns). doi: 10.4236/fns.2014.51011.