An overview of Surgical treatment of gastric cancer in Albania
Dea Maliqi,1 Arvit Llazani,2 Prof. Arvin Dibra2
1 Catholic University Our Lady of Good Counsel, Tirana, Albania
2 First Clinic of Surgery, Department of Surgery, University Hospital Center “Mother Theresa” Tirana, Albania
Introduction
Gastric cancer (GC) ranks as the fifth most prevalent cancer globally, with approximately one million new cases reported worldwide in 2020.1 According to Globocan 2020 data gastric cancer ranks as the fourth most prevalent cancer in Albania.11 The predominant histological type is adenocarcinoma (95% of GC cases) followed by gastrointestinal stromal tumor (GIST), leiomyoma, lymphoma, and neuroendocrine tumor.2
The primary curative option for GC is surgery. Gastrectomy with D2 lymphadenectomy is considered the standard surgical approach for locally advanced GC. The surgical options of gastric cancer are followed by reconstruction techniques achieving optimal resection margins, pathologic staging, and harvesting an adequate number of lymph nodes, significantly influence patient postoperative functional status. Despite notable improvements in surgical approaches for gastric cancer over recent decades, the mortality rate associated with this disease remains elevated.
The purpose of this study is to describe our surgery experience for GC.
Materials and methods
This retrospective analysis was performed on a total of 80 patients that underwent surgical procedures for gastric cancer (GC) at the First Service of General Surgery, UHC "Mother Theresa" in Tirana, Albania between January and December 2019.
The analysis includes demographical characteristics, tumor staging, and surgical procedures performed, with a particular focus on patients treated with curative surgical intent. The information was deducted from the clinical file of patients.
Preoperative staging procedures included abdominal and pelvic computed tomography, upper digestive endoscopy, and laboratory tests.
Patients involved in the study were those who had previously undergone diagnostic or endoscopic procedures and subsequently proceeded to resection. The type of gastric resection (total or subtotal) was based on the tumor's location, aiming to achieve a tumor-free proximal margin (R0).
Staging was based on the Eighth Edition of the TNM system by the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC).3
The surgical approach and the extent of lymphadenectomy adhered to the guidelines recommended by the Japanese Gastric Cancer Association 6th Edition.4
Patients with tumors originating outside the stomach or those undergoing gastric surgical procedures for benign conditions, such as peptic ulcers or gastrostomy for other reasons were excluded from this study.
Results
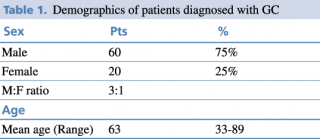
Demographical results are presented in Table 1. The patients included in the study hail from various regions of Albania, although a significant portion originates from the capital city, Tirana. It's noteworthy to mention that the majority of the participants are ethnically Albanian, belonging to the Caucasian ethnic group.
Table 1. Demographics of patients diagnosed with GC
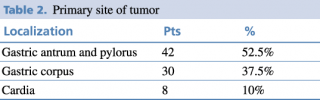
The patients were divided into three groups according to the primary site of the tumor as shown in Table 2.
Table 2. Primary site of tumor
Gastric adenocarcinoma, the most frequent histological type was diagnosed in 63 patients, whereas nonadenocarcinoma primary gastric tumors were detected in 11 patients in total as presented in Table 3.
Table 3. Histological types of GC
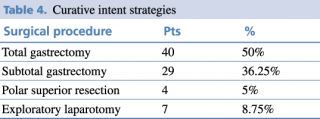
Of these patients, 91.3% were treated with curative intent, as shown in Table 4, while 8.7 % underwent palliative surgery as they were not considered candidable for surgery.
The reasons of inoperability were peritoneal carcinomatosis (P+), positive peritoneal cytology (CY+), distant lymph node metastasis (LN+), multiple liver metastasis (H+), distant organs metastasis (brain, bone, ecc.) and palliative procedures were done 3 gastrostomies, 3 gastroenteroanastomosis.
Table 4. Curative intent strategies
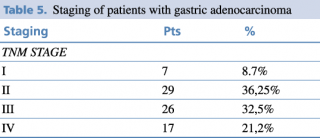
Regarding the UICC TNM stage classification, TNM, advanced stage was the most frequent as shown in Table 5. According to the histopathological findings, 32.5% of the patients are in stage III and 21,2 % stage IV.
Mean number of lymph nodes harvested are 15 (range min 12 – max 35) LN.
Table 5. Staging of patients with gastric adenocarcinoma
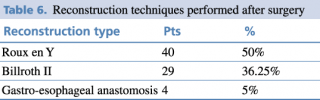
Reconstruction techniques as shown in Table 5 were performed depending on the type of surgery.
Table 6. Reconstruction techniques performed after surgery
Discussions
Typically, there is a higher incidence of gastric cancer in men compared to women, as indicated by the demographic data of our study which is concordant with other studies.5 Other studies suggest that the gender disparity is not statistically significant among individuals below the age of 45.6 The difference is observed in older age groups, reaching its peak in the 65-69 age range, where the male-to-female ratio is reported to be 3:1. An increase in the incidence of gastric cancer in women was observed when compared to studies conducted in previous years.7
The most common site was the distal stomach, precisely the antrum-pyloric region. This distribution aligns with other studies8 and is linked to major risk factors, including H. pylori infection, smoking habit, and excessive daily intake of salt.
Histopathological findings indicated that most patients were diagnosed in stage III and IV (more than 50% of the patients). The patients are in advanced stage when they present in medical services.
Common surgical procedures included total gastrectomy with D2 lymphadenectomy, followed by subtotal gastrectomy. The choice of gastric resection depended on tumor location and the ability to achieve R0-margin free of tumor. Total gastrectomy was prevalent in tumors of the stomach’s body, while subtotal resection was performed in the antrum-pyloric region and polar superior for cardia region in selected patients.
Lymphadenectomy is now considered standard for treating gastric cancer and is recommended in various national guidelines for patients with advanced gastric cancer.9 A Cochrane systematic review found that D2 lymphadenectomy led to significantly better disease-specific survival compared to D1 lymphadenectomy, despite a higher mortality rate in the D2 group. However, no significant difference was observed in the disease-free interval between the two groups. The Japan Clinical Oncology Group (JCOG) trial 9501 revealed that D3 lymphadenectomy, which includes para-aortic lymph node dissection, resulted in higher morbidity rates but did not significantly improve overall 5-year survival or local recurrence compared to D2 lymphadenectomy. Analysis of the Surveillance, Epidemiology, and End-Results (SEER) database suggests that removing more than 15 lymph nodes during gastrectomy for gastric cancer can improve survival.12,13,14,15
The mean number of lymph nodes harvested in this study was 15 which follows the NCCN guidelines that recommend D2 lymphadenectomy or a minimum recovery of 15 lymph nodes for safety and an average of 29 retrieved lymph nodes for adequacy.10
The debate over extended resection (D2 resection with splenectomy and distal pancreatectomy) for advanced gastric cancer, initially performed by Japanese surgeons, has persisted for decades. While early reports suggested improved survival, large prospective randomized controlled trials failed to demonstrate a significant survival benefit. Patients undergoing splenectomy and distal pancreatectomy experienced higher morbidity, mortality, and longer hospital stays. In a study by Otsuji et al., out of 128 patients undergoing total gastrectomy for gastric adenocarcinoma, pancreatosplenectomy (PS) and splenectomy (S) were associated with higher morbidity and mortality rates, primarily due to pancreatic fistula occurrence.16,17,18,19
Conclusions
Despite a decrease in gastric cancer incidence across Europe in the past few decades, it remains a relatively common tumor in Albania.
In the present study, we reported a broad view of GC surgical treatment in the Service of First Clinic in Mother Teresa Hospital.
At present, the primary curative treatment for gastric cancer is surgical resection, in advanced gastric cancer preoperatory neoadjuvant chemotherapy is indicated.
This study provides an indicator of the importance of a proper staging of gastric cancer following the AJCC Staging System which is one of the main factors determining the treatment type.
The study suggests that gastric surgery with D2 lymphadenectomy could be effectively performed in patients with defined tumor margins and with low operative risk. The positivity to distant metastases could lead to inoperable cases that terminate with exploratory laparotomy or nutritional jejunostomy.
References
- Ferlay J, Ervik M, Lam F. GLOBOCAN 2018, Global Cancer Observatory: Cancer Today. LyonFrance: International Agency for Research on Cancer. Published online 2021.
- Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. World Health Organization, 2010.
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-Based to a More “Personalized” Approach to Cancer Staging: The Eighth Edition AJCC Cancer Staging Manual. CA Cancer J Clin. 2017;67(2):93-99. doi:10.3322/caac.21388
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 2023;26(1):1-25. doi:10.1007/s10120-022-01331-8
- Wong MCS, Huang J, Chan PSF, et al. Global Incidence and Mortality of Gastric Cancer, 1980-2018. JAMA Netw Open. 2021;4(7):e2118457. doi:10.1001/jamanetworkopen.2021.18457
- Lou L, Wang L, Zhang Y, et al. Sex Difference in Incidence of Gastric Cancer: An International Comparative Study Based on the Global Burden of Disease Study 2017. BMJ Open. 2020;10(1):e033323.
- Lagergren F, Xie SH, Mattsson F, Lagergren J. Updated Incidence Trends in Cardia and Non-Cardia Gastric Adenocarcinoma in Sweden. Acta Oncol. 2018;57(9):1173-1178. doi:10.1080/0284186X.2018.1457797
- D’Elia L, Rossi G, Ippolito R, Cappuccio FP, Strazzullo P. Habitual Salt Intake and Risk of Gastric Cancer: A Meta-Analysis of Prospective Studies. Clin Nutr. 2012;31(4):489-498. doi:10.1016/j.clnu.2012.01.003
- Kulig J, Popiela T, Kolodziejczyk P, Sierzega M, Szczepanik A, Polish Gastric Cancer Study Group. Standard D2 Versus Extended D2 (D2+) Lymphadenectomy for Gastric Cancer: An Interim Safety Analysis of a Multicenter, Randomized, Clinical Trial. Am J Surg. 2007;193(1):10-15.
- Woo Y, Goldner B, Ituarte P, et al. Lymphadenectomy with Optimum of 29 Lymph Nodes Retrieved Associated with Improved Survival in Advanced Gastric Cancer: A 25,000-Patient International Database Study. J Am Coll Surg. 2017;224(4):546-555. doi:10.1016/j.jamcollsurg.2016.12.015
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. doi:10.3322/caac.21660
- Mocellin S., McCulloch P., Kazi H., Gama-Rodrigues J.J., Yuan Y., Nitti D. Extent of Lymph Node Dissection for Adenocarcinoma of the Stomach. Cochrane Database Syst. Rev. 2015 doi: 10.1002/14651858.CD001964.pub4. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Fujimura T., Nakamura K., Oyama K., Funaki H., Fujita H., Kinami S., Ninomiya I., Fushida S., Nishimura G., Kayahara M. Selective Lymphadenectomy of Para-Aortic Lymph Nodes for Advanced Gastric Cancer. Oncol. Rep. 2009; 22:509–514. doi: 10.3892/or_00000464. [PubMed] [CrossRef] [Google Scholar]
- Schwarz R.E., Smith D.D. Clinical Impact of Lymphadenectomy Extent in Resectable Gastric Cancer of Advanced Stage. Ann. Surg. Oncol. 2007; 14:317–328. doi: 10.1245/s10434-006-9218-2. [PubMed] [CrossRef] [Google Scholar]
- Ye J., Ren Y., Dai W., Chen J., Cai S., Tan M., He Y., Yuan Y. Does Lymphadenectomy with at Least 15 Perigastric Lymph Nodes Retrieval Promise an Improved Survival for Gastric Cancer: A Retrospective Cohort Study in Southern China. J. Cancer. 2019; 10:1444–1452. doi: 10.7150/jca.28413. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Kano Y., Ohashi M., Ida S., Kumagai K., Makuuchi R., Sano T., Hiki N., Nunobe S. Therapeutic Value of Splenectomy to Dissect Splenic Hilar Lymph Nodes for Type 4 Gastric Cancer Involving the Greater Curvature, Compared with Other Types. Gastric Cancer. 2020; 23:927–936. doi: 10.1007/s10120-020-01072-6. [PubMed] [CrossRef] [Google Scholar]
- Maruyama K., Okabayashi K., Kinoshita T. Progress in Gastric Cancer Surgery in Japan and its Limits of Radicality. World J. Surg. 1987; 11:418–425. doi: 10.1007/BF01655804. [PubMed] [CrossRef] [Google Scholar]
- Lo S., Wu C., Shen K., Hsieh M., Lui W. Higher Morbidity and Mortality After Combined Total Gastrectomy and Pancreaticosplenectomy for Gastric Cancer. World J. Surg. 2002; 26:678–682. doi: 10.1007/s00268-001-0289-8. [PubMed] [CrossRef] [Google Scholar]
- Otsuji E., Yamaguchi T., Sawai K., Okamoto K., Takahashi T. Total Gastrectomy with Simultaneous Pancreaticosplenectomy оr Splenectomy in Patients with Advanced Gastric Carcinoma. Br. J. Cancer. 1999; 79:1789–1793. doi: 10.1038/sj.bjc.6690285. [PMC free article] [PubMed] [CrossRef] [Google Scholar]