Type 2 Diabetes (T2D) and Arsenic at low concentrations: are there any real associations? A systematic review.
Sandro Mancinelli,1 Patrizia De Filippis,1 Alessandra Messina,1 Maria Spoto,1 Stefano Orlando,1 Laura Morciano,1* Leonardo Palombi1 and Francesca Lucaroni1
1Department of Biomedicine and Prevention, University of Rome Tor Vergata
*Correspondence: Dr. Laura Morciano
1. Introduction
Longlife exposure to metals – in particular, arsenic – has been identified as a risk factor for a number of neoplastic and nonneoplastic diseases.
The global burden of people exposed to arsenic levels over the threshold value of 10µg/L, determined by the US Environmental Protection Agency in 2001, has been estimated to be around two hundred and twenty six million people.1 World regions with higher levels of this metalloid – with mean concentrations higher than 150µg/L and, therefore, significant human exposure – have been identified in Bangladesh, India, Taiwan, China, Mexico, Argentina, Chile, the USA and some European areas.2
Arsenic and its compounds have been demonstrated to be risk factors in a number of chronic diseases: cancer, cardiovascular diseases, peripheral neuropathy, Type 2 diabetes and skin lesions.3 Both incidence and prevalence of Type 2 diabetes (T2D) are rapidly increasing worldwide, also in emerging economies, characterizing a true epidemic. Although much is already known about this disease, classical theories on its pathogenesis don’t fully explain the present situation. Current scientific research has focused on environmental factors in order to fill these knowledge gaps.
In Diabetes, chronic exposure to arsenic and its compounds induces both βcell dysfunction and insulin resistance, through a mechanism of oxidative stress,4 which provokes a progressive βcell failure, and a decrease in the nuclear receptor PPARµ (Peroxisome proliferatoractivated receptor gamma),5 involved in the regulation of fatty acid storage and glucose metabolism, which might reduce the sensitivity of insulin responsible for insulin activation.6
At high doses, in case of acute intoxication, arsenic is even able to interfere with ATPdependent insulin secretion and with glucose transporters (GLUTs), due to its ability to be the shadow element of phosphate.7
At the same time, studies evaluating the role of exposure in the range of low to moderate levels of this metalloid – respectively <50 μg/L and 50150 μg/L, according to the National Research Council definition8 – still give inconclusive results, probably because of the poor robustness of most studies to date.
However, in recent years, the growing interest in metalloid pollution has addressed the implementation of a number of studies evaluating the potential role of lowdose exposure. In 2008, the University of Chicago coordinated a longitudinal study, the Health Effects of Arsenic Longitudinal Study (HEALS), which evaluated arsenic toxicity in more than 20,000 people exposed to lowmoderate levels of Arsenic (0.1 to 864 μg/L, mean 99 μg/L) in Araihazar, Bangladesh. The results of this study suggested an increased risk of skin lesions, hypertension, neurological dysfunctions, and mortality for all causes.9 In addition to this, another study on lowmoderate arsenic exposure was carried out in 2013 under the supervision of the Johns Hopkins Bloomberg School of Public Health on a cohort of nearly 4000 native Americans (The STRONG HEART STUDY). This study demonstrated an association between chronic exposure to low dose arsenic by the mean of drinking water and increased risk for cardiovascular disease and lung, prostate, and pancreatic cancer.10
2. Materials and Methods
The authors referred to Preferred Reporting Items for Systematic Reviews and MetaAnalyses (PRISMA) guidelines to achieve this systematic review.
2.1. Eligibility criteria:
2.1.1. Type of studies:
Only original researches (no reviews, editorials, or research letters) concerning humans, published from 1st of January 2005 to present, and investigating the existence of association between low arsenic exposure and the developing of Type 2 Diabetes (T2D), were included.
2.1.2. Type of participants:
Adults exposed to low levels of arsenic by the mean of drinking water were considered. Low levels were defined as total (or inorganic) arsenic mean concentration lower than 100µg/L or 300 µg/g creatinine. This cut off level was selected basing on the Andra group study, published in 2013.11
2.1.3. Outcome measures:
Primary outcome was the developing of Type 2 Diabetes, measured using American Diabetes Association (ADA) criteria. Researches adding further criteria of diagnosis to ADA ones, were also included.
2.2. Exclusion criteria:
Studies with no available abstract, and those concerning occupational exposure to arsenic, were also excluded, although they met the preview eligibility criteria.
2.3. Data sources:
A systematic review of articles, written in all languages, was achieved on 22nd of April 2016 using Pubmed, Scopus and Web of Science.
Additionally, two further works – coming from institutional publications and the examination of references of the previews articles – were included.12, 13
Two investigators (F.L. and S.M.) independently extracted the articles that met the eligibility criteria. Study selection was performed with the inclusion of all relevant available studies containing the following terms: “arsenic”* AND “diabetes” as Mesh term, both AND and without “low”; “arsenic”* AND “T2D”, both AND and without “low”.
The preview searches were achieved within title and abstract for Pubmed, within title, abstract and keywords for Scopus, and only within title for Web of Science.
The disagreements were resolved by consensus.
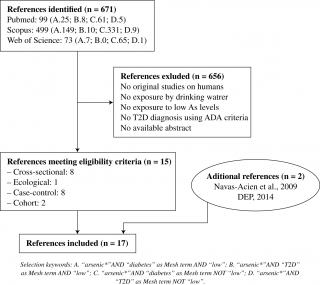
The research, undertaken following the preview search criteria, released an overall number of 671 studies. After the exclusion of duplicates and of studies that didn’t meet the eligibility criteria, 15 works were identified, as summarized in Figure n.1, representing the study selection process. In addition to the fifteen published articles eligible for this systematic review, two further works – coming from institutional publications and the examination of references of the previews articles – were added, for an overall number of 17 reviews analyzed for qualitative synthesis.
Quality assessment of the included studies was performed using the specific NHLBI Assessment Tools.14
2.4. Statistical methods:
Statistical measures of association (Odds ratio, Hazard ratio, Standardized Mortality ratio) were used to synthesize results of the included studies. In case of more than one model of adjustment, the authors selected the one adjusted for the most number of covariates.
3. Results
The search on Medline, Scopus and Web of Science provided a total of 671 citations. After adjusting for duplicates and discarding all the studies that did not meet the eligible criteria (see Figure n. 1), a total of 15 studies were included.
All fifteen studies selected were original reviews, written in any language, and published from 1st of January 2005 to present. They all concerned human exposures at low doses of arsenic – with mean concentrations ≤100µg/L or ≤300µg/g creatinine – and development of Type 2 Diabetes (T2D), measured as primary outcome. In addition to eligible articles, two additional studies – coming from institutional publications and the examination of references of the previews articles – were considered, for an overall number of 17 reviews analyzed for qualitative synthesis.
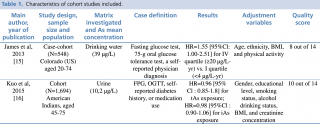
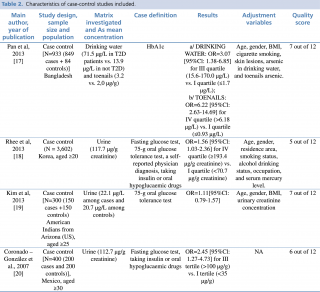
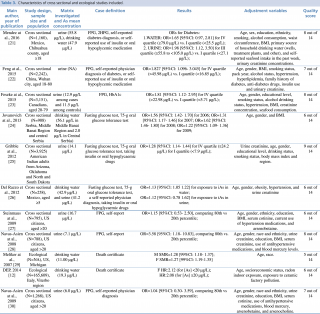
Main characteristics of the included studies are summarized in Table n. 1, 2 and 3.
The most part of them presented only one biomarker of exposure: inorganic arsenic (iAs) or total arsenic (tAs) in urine was investigated in almost 60% of reviews (N=10); while in three studies exposure was measured using drinking water samples. Only three studies out of seventeen examined more than one biomarker of exposure: arsenic concentration in urine and water21, 26 or arsenic in drinking water and in toenails.17
Among the seventeen studies examined, one out of three (N = 11) had a cross sectional or ecological design, these ones using T2D mortality ratio as primary outcome.
The most recent publication aimed at identifying associations between T2D and chronic arsenic exposure was achieved by Mendez et al21 on a sample of 1,160 men and women, aged ≥ 18 and living in the Chihuahua county, Mexico. This crosssectional study pointed out that chronic exposure to high arsenic concentrations was associated with increased odds of diabetes in case of urinary exposure ≥55.8 μg/L. Otherwise, exposure by the mean of drinking water didn’t show any statistically significant associations with T2D.
Contrary from Mendez, although sampling a similar population – Mexican men and women, with similar exposure to arsenic, both in urine and in drinking water – another group of authors26 found increased risk of developing diabetes only if biomarker of exposure was measured in drinking water {OR =1.13 [95% CI: 1.05 1.22]}, and not in urine {OR= 1.12 [95% CI: 0.78 1.62]}.
All the crosssectional studies investigating arsenic concentration in urine as biomarker of exposure22, 23, 25, 27, 28, 30 evidenced a significant association with diabetes, with odds of disease swinging between 1.28 [95%CI:1.141.44] of Gribble et al., 2012 and 3.58 [95%CI:1.1810.83] of NavasAcien et al., 2008.
NavasAcien’s group published two crosssectional studies,28,30 both achieved inside the National Health and Nutrition Examination Survey (NHANES), a program begun in the early 1960s, thanks to a collaboration between the Center for Disease Control and Prevention (CDC) and the National Center for Health Statistics (NCHS). This, program-aimed at assessing the health and nutritional status of adults and children in the United States, combined interviews and physical examinations.
From 2003 to 2006 NavasAcien group followed two cohort of US men and women, respectively of 788 and 1,279 people, aged more than 20, exposed to mean inorganic arsenic concentrations slightly higher than 7 μg/L.
The first study showed increased odds of T2D of 258% [OR=3.58 (with 95%CI: 1.1810.83)]; in the second study the odds risk fluctuated from OR=2.60 [95% CI: 1.12 6.03], without correction for arsenobetaine, to OR=4.26 [95% CI: 0.83 21.8], after correction for arsenobetaine. The model proposed to adjust for arsenobetaine has been largely debated during the successive years. In 2009 Steinmaus et al. published a reanalysis of NavasAcien group’s study, with different adjustment models. Comparing 80th vs 20th percentile of inorganic arsenic concentrations in urine, they found no statistically significant odds of diabetes: OR=1.15 [95% CI: 0.53–2.50], suggesting no identifiable association between T2D and urinary inorganic arsenic using NHANES data.
The NHANES study is just one of the number of scientific studies carried out on low to moderate levels of arsenic exposure (<150 µg/L) worldwide.
In 2015 Kuo et al, published a cohort study with 1,694 people, coming from the STRONG HEART STUDY (SHS) cohort, meanly exposed to arsenic concentrations slightly greater than law levels (with a median concentration of 10.2 µg/L).16 The SHS is the largest epidemiologic study ever undertaken on American Indians, with the support of the National Heart, Lung, and Blood Institute (NHLBI). Since 1998, it has examined cardiovascular diseases and their main risk factors among American Indians, both men and women. From 1989 to 1999 Kuo and his group followed a cohort of 1,694 American Indians, aged 4575. Evaluating exposure to total arsenic in urine, he identified no association with a higher diabetes incidence, with an HR= 0.96 [95%CI: 0.85–1.08].
Among the seventeen included reviews, only another cohort study met our eligible criteria, the one achieved by James et al. on a sample of 548 adults living in Colorado, US. They had a mean exposure to 39 μg/L of inorganic arsenic in drinking water is statistically associated to T2D, with a risk of developing the disease of 55% higher than standard, defined as exposure to iAs in drinking water <4μg/Lyr {HR=1.55 [95%CI: 1.002.51]}.
Similarly to the studies undertaken by Kuo and James, also the casecontrol studies undertaken on the issue17, 18, 19, 20 show an extreme heterogeneity of results. In 2013 Rhee et al. achieved a study based on the Korea National Health and Nutrition Examination Survey (KNHANES) population. Similarly to the American NHANES, this is a national program designed to assess the health related behavior, health condition, and nutritional state of Koreans. Rhee and his group evidenced that a mean exposure to total arsenic concentration of 117.7 μg/g creatinine in urine is associated with T2D, with odds of disease increased of 56% {OR = 1.56 [95%CI: 1.032.36]}, considering the IV quartile (exposure to tAs≥ 193.4 μg/g creatinine) vs. I quartile (exposure to tAs < 70.7μg/g creatinine).
The same trend of Rhee’s work is followed by one further study, achieved by Pan et al in 2013. Pan and his group examined a sample of 933 men and women – 849 cases and 84 controls – selected from general population in Bangladesh. Investigating total arsenic levels as biomarkers of exposure, assessed that also exposure to arsenic mean concentrations <100 μg/L in drinking water and toenails increase odds of diabetes: OR= 3.07 [95%CI: 1.386.85] for water exposure and OR= 6.22 [95%CI: 2.6314.69] for toenails.
Some years earlier, in 2007, also CoronadoGonzález and his group20 reached similar conclusions, finding an OR=2.45 [95%CI: 1.27–4.73] for exposures to urinary arsenic concentrations higher than 100μg/g creatinine, if compared with the reference group, exposed to tAs levels <35 μg/g creatinine.
Differently from results by Rhee, Pan and CoronadoGonzález, Kim and his group’s study19 pointed out there was no significant difference among case and control groups in case of exposure to arsenic low concentrations, slightly higher than 20 μg/L both in case and controls.
The majority of studies performed outside of standardized national protocols also showed a somehow significant association between arsenic exposure and risk of T2D. An ecological study – carried out by Meliker29 in Michigan, US, in 2007 demonstrated a higher standardized mortality ratio for mean exposure of 11.00 μg/L, both in men and in women: SMR 1.28 (99% CI: 1.18–1.37) in men and SMR 1.27 [99% CI:1.19–1.35] in women.
Seven years later, a second ecological study, undertaken by the Italian Department of epidemiology – Lazio Region (DEP) analyzed data from 1990 to 2010, and published the only study carried out on this issue in Italy.12 The risk of developing chronic diseases in a large population (N=165,609) of residents in central Italy, chronically exposed to arsenic at higher concentrations than legal limits was investigated. Data from mortality records was matched with total arsenic exposure, estimating combined geocoded past and present residential addresses of cohort participants. Hazard ratio, adjusted for the main confounders such as age, gender, socioeconomic status, but also radon indoor exposure and exposure to ceramic factory pollution was higher than normal, but just in women. The risk of T2D fluctuated from 108% to 112% higher in the two different groups of exposure considered: HR 2.12 for [As]<20 μg/L exposure and HR 2.08 for [As]≥20 μg/L exposure.
The existence of a statistically significant association between T2D and exposure to low to moderate arsenic concentrations was confirmed by Wang et al., who published in the same year a wide meta analysis,31 evaluating articles published between 1990 and 2013, with a cumulative sample size of 2,243,745 for iAs in drinking water and 21,083 participants for total arsenic (tAs) in urine. Although not specifically targeted to low to moderate arsenic levels (<150 μg/L) of exposure, the metaanalysis showed an increased in the relative risk for T2D both for iAs and total As exposure. The analysis pointed out a relative risk of 1.75 (95%CI 1.20 to 2.54) of developing Type 2 Diabetes for the highest versus the lowest category of inorganic Arsenic exposure level in drinking water. According to the doseresponse analysis, risk of diabetes was 13% (CI 95% 1.001.27) higher for every 100 mg/L raise of inorganic arsenic. A statistically significant association with T2D was also found with total Arsenic in urine as a biomarker of exposure, with an increase in 28% of risk of developing the disease [RR 1.28 (95%CI 1.141.44)].
4. Discussion
In the last decades, many studies focused on the relationship between low arsenic exposure and development of Type 2 Diabetes in general population, but always with inconsistent results. The only systematic review achieved on this issue, undertaken by NavasAcien group32 and published in 2006, found no evidence of association, although mainly due to the limited quality of available evidences.
From 2005 to 2016, as evidenced by our review, the number of original studies assessing the association between T2D and arsenic exposure <100 µg/L deeply increased, although with weak consistency results. Indeed, most part of studies focusing on low exposure were cross sectional or ecological. Focusing on a large sample of general population, they evidenced a slightly increased risk of developing Type 2 Diabetes, both if biomarker of exposure was measured in urine and in drinking water, with risk measures fluctuating from 1.2825 and 3.5828 for urine, and from 1.1326 and 1.2224 for drinking water.
It should be pointed out that odds of disease higher than 258% in people exposed to arsenic in NavasAcien study reflect the inclusion of arsenobetaine in adjustment model that, as suggested by Steinmaus et al., might condition the result. Furthermore, the wide confidence interval of the risk measure [95%CI: 1.1810.83], comparing 80th vs 20th percentile, might be explained by the unrecognized existence of different subpopulations with diverse peculiarities that could act as possible confounders.
Also the two selected ecological works evidenced, for mean exposure to arsenic concentrations <20 μg/L, an increased risk of mortality for diabetes, both for men and women in Meliker study {MSMR=1.28 [99% CI: 1.18–1.37]; F SMR=1.27 [99% CI:1.19–1.35]}, and only for women in the Italian study, DEP 2014 {F HR:2.12 (for [As]<20 μg/L); HR: 2.08 (for [As]≥20 μg/L)}.
Within the five incidence casecontrol studies that met our eligibility criteria only the one with the smallest sample size19 demonstrated no evidence of association. All the other studies, in particular the two with larger sample size,18,17 showed an increased risk of T2D. In their study based on a population of 3,602 adults, resident in Korea, Rhee and his group, evidenced an increased odds of developing diabetes, if arsenic was measured in urine, equal to 56%: OR = 1.56 [95%CI:1.032.36] for IV quartile (≥ 193.4 μg/g creatinine) vs. I quartile (< 70.7μg/g creatinine).
In 2013 Pan and his group, thanks to their studies analyzing arsenic in drinking water and toenails samples, demonstrated an increased risk of T2D also in case of exposure to low arsenic concentrations in household water, with an OR= 3.07 [95%CI: 1.386.85] for III quartile (As: 15.6–170.0 μg/L) compared with I quartile (As≤1.7 μg/L).
In addition to the studies cited above, only two prospective cohort studies15,16 met our selection criteria. These works, conducted on different populations – American Indians aged 4575, and general population, resident in Colorado (US) and aged 2074 – and using diverse biomarkers of effect (urine, drinking water), gave different results: a statistically significant association between total Arsenic exposure and Type 2 Diabetes at exposures <100µg/L was identified in the study with smaller sample.15 At mean arsenic exposure of 39 μg/L, the 548 people enrolled showed an increased risk of developing T2D equal to 55% after adjustment for Age, ethnicity, BMI, and physical activity. On the contrary, Kuo and his group, measuring the total arsenic levels in urine samples of 1,694 American Indians, found no significant association between diabetes and low arsenic exposure.
5. Conclusions
Exposure to arsenic and its compounds by means of drinking water has been largely demonstrated to be an important risk factor for Type 2 Diabetes at high levels of chronic exposure (>150 μg/l). The effects of exposure to low/moderate concentrations of this metalloid remain substantially controversial, in spite of the plethora of scientific studies that have been produced in the last decades. This might depend on different contemporary reasons: the poor robustness and the extreme heterogeneity of the available study designs, as well as the existence of complex interactions that could modify the effect of exposure to arsenic and its compounds. We could hypothesize that different factors – genetics, environmental, nutritional, lifestyle – might play an important role, competing with the arsenic mode of action and changing its effects on human health. These contradictory results could be also explained by the existence of an overlap of different subpopulations, not considered separately in some of these studies. This hypothesis might particularly contribute to explain the substantial increase in risk measures, combined with a wide range of confidence intervals, recorded in some of the largest studies, as NavasAcien’s.
Therefore, further investigation is still necessary. More prospective studies should be conducted, in particular considering different subpopulations, in order to minimize possible confounders. For example, immigrants coming from countries with higher arsenic environmental levels might be selected as specific subgroups for investigation.
Irrespective of the reasons why results had no consistency, understanding the effects of low to moderate arsenic exposure is extremely urgent because of the worldwide environmental diffusion of this metalloid and the absence of effective treatment against acute and chronic intoxications. If arsenic toxicity at low to moderate levels of exposure was confirmed, this would expand the population at risk to two hundred and twenty six million people worldwide, including arsenic exposures coming both from geological and anthropogenic sources in water and food, with increased public health worldwide, both in terms of morbidity/mortality and economic expenditures.
References
- Murcott S. Arsenic Contamination in the World: An International Sourcebook. London: IWA Publishing; 2012.
- Lucentini L, Aureli F, Crebelli R, Cubadda F, D’Amato M, La Sala L, Ottaviani M, Veschetti E, Mantovani Esposizione ad arsenico attraverso acqua ed alimenti in aree a rischio: il caso del Lazio. Available from: http://www.iss.it/binary/publ/cont/onlinefebbr2013.pdf. [Accessed 29th Dec 2014].
- Mancinelli M, Lucaroni F, Borgiani P, Ciccacci C, Palombi L, De Filippis P. Low Level Exposure to Arsenic in Drinking Water: A Review on Action Mechanism, Health Effects and Biomarkers. The International Journal of Scientific Research. 2015; 4 (9). Journal DOI: 10.15373/22778179.
- Fu J, Woods CG, YehudaShnaidman E, Zhang Q, Wong V, Collins S, Sun G, Andersen ME, Pi J. Low Level Arsenic Impairs GlucoseStimulated Insulin Secretion in Pancreatic Beta Cells: Involvement of Cellular Adaptive Response to Oxidative Stress. Environmental Health Perspectives. 2010; 118(6).
- Yadav S, Anbalagan M, Shi Y, Wang F, Wang H. Arsenic Inhibits the Adipogenic Differentiation of Mesenchymal Stem Cells by DownRegulating Peroxisome ProliferatorActivated Receptor Gamma and CCAAT EnhancerBinding Proteins. Toxicol In Vitro. 2013; 27(1): 2119.
- Singh AP, Goel RK, Kaur T. Mechanisms Pertaining to Arsenic Toxicity. Toxicol Int. 2011; 18(2): 87–93.
- Tseng CH. The Potential Biological Mechanisms of ArsenicInduced Diabetes Mellitus. Toxicol. Appl. Pharmacol. 2004; 197: 67–83.
- National Research Council (NRC). Critical Aspects of EPA’s IRIS Assessment of Inorganic Arsenic. Interim Report; The National Academic Press: Washington DC, USA, 2014.
- Ahsan H, Chen Y, Parvez F, Argos M, Hussain AI, Momotaj H, Levy D, van Geen AHG, Graziano J. Health Effects of Arsenic Longitudinal Study (HEALS): Description of a Multidisciplinary Epidemiologic Investigation. J. Expo. Sci. Environ. Epidemiol. 2006; 16(2): 191205.
- García Esquinas E, Pollán M, Umans JG, Francesconi KA, Goessler W, Guallar E, Howard B, Farley J, Best LG, NavasAcien A. Arsenic Exposure and Cancer Mortality in an USBased Prospective Cohort: The Strong Heart Study. Cancer. Epidemiol Biomarkers Prev. 2013; 22(11): 1944–1953.
- Andra SS, Makris KC, Christophi CA, Ettinger AS. Delineating the Degree of Association Between Biomarkers of Arsenic Exposure and Type 2 Diabetes Mellitus. International Journal of Hygiene and Environmental Health. 2013; 216(1): 3549.
- Dipartimento Epidemiologia del S.S.R. Regione Lazio (DEP), 2014. Valutazione Epidemiologica degli effetti sulla salute in relazione alla contaminazione da arsenico nelle acque potabili. Studio di coorte di mortalità nella popolazione residente in provincia di Viterbo, 19902010.
- Navas Acien A, Silbergeld EK, PastorBarriuso R, Guallar E. Arsenic Exposure and Prevalence of Type 2 Diabetes: Updated Findings from the National Health Nutrition and Examination Survey, 20032006. Epidemiology. 2009; 20(6): 816–e2, doi:10.1097/EDE.0b013e3181afef88.
- NHLBI. Study quality assessment tools. Available from: http://www.nhlbi.nih.gov/healthpro/guidelines/indevelop/cardiovascularriskreduction/tools [Accessed 15th September 2016]
- James KA, Marshall JA, Hokanson JE, Meliker JR, Zerbe GO, Byers TE. A CaseCohort Study Examining Lifetime Exposure to Inorganic Arsenic in Drinking Water and Diabetes Mellitus. Environmental Research. 2013; 123: 33–38.
- Kuo CC, Howard BV, Umans JG, Gribble MO, Best LG, Francesconi KA, Goessler W, Lee E, Guallar E, NavasAcien A. Arsenic Exposure, Arsenic Metabolism, and Incident Diabetes in the Strong Heart Study. Diabetes Care. 2015; DOI: 10.2337/dc141641.
- Pan WC, Kile ML, Seow WJ, Lin X, Quamruzzaman Q, Rahman M, Mahiuddin G, Mostofa G, Lu Q, Christiani DC. Genetic Susceptible Locus in NOTCH2 Interacts with Arsenic in Drinking Water on Risk of Type 2 Diabetes. PLoS One. 2013; 8(8), e70792.
- Rhee SY, Hwang YC, Woo JT, Chin SO, Chon S, Kim YS. Arsenic Exposure and Prevalence of Diabetes Mellitus in Korean Adults. J Korean Med Sci. 2013; 28: 861868, http://dx.doi.org/10.3346/jkms.2013.28.6.861.
- Kim NH, Mason CC, Nelson RG, Afton SE, Essader AS, Medlin JE, et al. Arsenic Exposure and Incidence of Type 2 Diabetes in Southwestern American Indians. Am J Epidemiol. 2013; 177:962–969.
- Coronado González JA, Del Razo LM, GarcíaVargas G, SanmiguelSalazar F, Escobedode la Peña J. Inorganic Arsenic Exposure and Type 2 Diabetes Mellitus in Mexico. Environ.Res. 2007; 104:383– 389. doi:10.1016/j.envres.2007.03.004.
- Mendez MA, GonzalezHorta C, SanchezRamirez B, BallinasCasarrubias L, Hernandez Ceron R, Viniegra Morales D. Chronic Exposure to Arsenic and Markers of Cardiometabolic Risk — A CrossSectional Study in Chihuahua, Mexico. Environ. Health Perspect. 2015; 124 (1):104111.
- Feng W, Cui X, Liu B, Liu C, Xiao Y. Association of Urinary Metal Profiles with Altered Glucose Levels and Diabetes Risk: A PopulationBased Study in China. PLoS ONE. 2015; 10(4): e0123742. doi: 10.1371/journal.pone.0123742.
- Feseke SK, StLaurent J, AnassourSidi E, Ayotte P, Bouchard M, Levallois P. Arsenic Exposure and Type 2 Diabetes: Results from the 2007–2009 Canadian Health Measures Survey. Health Promotion and Chronic Disease Prevention in Canada. Research, Policy and Practice. 2015; 35 (4).
- Jovanovic D, RasicMilutinovic Z, Paunovic K, Jakovljevic B, Plavsic S, Milosevic J. Low Levels of Arsenic in Drinking Water and Type 2 Diabetes in Middle Banat Region, Serbia. Int J Hyg Environ Health. 2013; 216(1): 505, doi: 10.1016/j.ijheh.2012.01.001.
- Gribble MO, Howard BV, Umans JG, Shara NM, Francesconi KA, Goessler W, Crainiceanu CM, Silbergeld EK, Guallar E, and NavasAcien A. Arsenic Exposure, Diabetes Prevalence, and Diabetes Control in the Strong Heart Study. American Journal of Epidemiology. 2012; 176 (10): 865874.
- Del Razo LM, GarcíaVargas GG, Valenzuela OL, Hernández Castellanos E, SánchezPeña LC, Currier JM, Drobná Z, Loomis D, Stýblo M. Exposure to Arsenic in Drinking Water Is Associated with Increased Prevalence of Diabetes: A CrossSectional Study in the Zimapán And Lagunera Regions in Mexico. Environmental Health. 2011; 10: 73.
- Steinmaus C, Yuan Y, Liaw J, Smith AH. LowLevel Population Exposure to Inorganic Arsenic in the United States and Diabetes Mellitus: A Reanalysis. Epidemiology. 2009; 20: 807–815. doi: 10.1097/EDE.0b013e3181b0fd29.
- Navas Acien A, Silbergeld EK, PastorBarriuso R, Guallar E. Arsenic Exposure and Prevalence of Type 2 Diabetes in US Adults. JAMA. 2008; 300(7).
- Meliker JR, Wahl RL, Cameron LL, Nriagu JO. Arsenic in Drinking Water and Cerebrovascular Disease, Diabetes Mellitus, and Kidney Disease in Michigan: A Standardized Mortality Ratio Analysis. Environmental Health. 2007; 6:4, doi: 10.1186/1476069X64.
- Navas Acien A, Silbergeld EK, PastorBarriuso R, Guallar E. Rejoinder: Arsenic Exposure and Prevalence of Type 2 Diabetes: Updated Findings from The National Health Nutrition and Examination Survey, 2003– 2006. Epidemiology. 2009; 20: 816–820; discussion e811–812. doi: 10.1097/EDE.0b013e3181afef88
- Wang W, Xie Z, Lin Y, Zhang D. Association of Inorganic Arsenic Exposure with Type 2 Diabetes Mellitus: A MetaAnalysis. J Epidemiol Community Health. 2014; 68: 176–184, doi:10.1136/jech2013203114.
- Navas Acien A, Silbergeld EK, Streeter RA, Clark JM, Burke TA, Guallar E. Arsenic Exposure and Type 2 Diabetes: A Systematic Review of the Experimental and Epidemiological Evidence. Environ Health Perspect. 2006; 114: 641–648.